I was checking out the documentary about E=mc2 . The documentary attempts to bring to the fore the contribution of one of the greatest minds of the century, Albert Einstein, his equation relating mass, energy, and the speed of light. If we cut through the melodramatic presentation with the Victorian charms and the bourgeois society and its workings, rest of the documentary takes us through the most important developments that made Einstein's discovery possible. As Newton said 'standing on the shoulders of Giants', the documentary attempts to show the giants on whom Einstein stood to see farther than light, figuratively speaking.
The first important milestone happened to be the idea that various forms of energy though having an existence independent of their own, are inter-related and can be changed from one form to another. Heat in the steam, and electricity in the wire, and magnetism of the magnets were all examples of energy in the pristine form, all having a common undercurrent: "energy". Michael Faraday showed that electrical energy can be used to create magnetism in objects and vice versa. The theory of electro-magneitc waves and electromagnetic field came to be proposed for the first time.
The debate that then ensued was about the conservation of matter. That it can neither be created nor be destroyed. It was shown by Antoine Lavoisier, assisted by his wife Marie Anne, that water can be converted to steam, then passed through iron and then condensed at the other end. The liquid at the other end is water, but is lesser in mass than initial. The hydrogen gas collected along with the water in the container and the rust that had formed by reaction between iron and oxygen accounted for the missing mass.
The next crucial part was that energy was proportional to the square of the velocity. It was shown by a Dutch scientist that when a lead ball is dropped on to a box of clay, it makes 4 times deeper impression when dropped from twice the height. Thus providing evidence to the squared relation, though it went against the proposals of Newton at that time. It took a century to gain general acceptance among the scientific community. Major headway in these episodes was played by Gottfried Leibniz and Emilie du Chatelet. Emilie du Chatelet lead a prolific role in science apart from excelling in arts, and raising a family of 3 children. She translated the Newton's Principia in French.
We are then taken back to Faraday who had then proposed that light itself as a form of energy and hence an electromagnetic wave. It caused much furor as people were still grappling with the idea of the mysterious interplay between Electricity and Magnetism that Faraday himself had demonstrated earlier. Maxwell came along to show that mathematics did permit such a possibility. But then Maxwell also suggested that, to be consistent with his theory, even if one were to travel at the speed of light (690 million mile per hour), one would observe light traveling at the 690 million mile per hour away from them. If you travel next to a car at the same speed as that car, that car would then appear stationary to you. The proposal was counter-intuitive.
Einstein for his part, had to reconcile this fact. He realized that this is possible if the hands of the clock were to move slowly at higher speeds. Thus time was no more independent of the space, measured in watches. Space and time were now related. This dealt an incredible blow to 3 centuries of resolute faith in the scientific belief that time was an absolute.
Einstein began to wonder, what would happens if train were to be accelerated to the speed of light, and more fuel were added to it to accelerate it further? Will it travel at a speed greater than light? That's not possible. So, he concluded that the added energy must be converted to mass of the train to conserve energy.
Thus it allowed him to derive the relation between energy, mass, and velocity of light. E=mc^2
The documentary then goes on the show the meteoric rise of Einstein among the ranks and his proposal for special and general relativity of which a nice introduction can be found on shallowthgts.
So definitely get hold of the documentary and see it. You will learn about women who played very important role in science and have remained relatively unknown. You also get to see the coming together of important scientific developments, each one a truly great milestone.
IMDB: tt0476209
(an updated repost from the past)
Showing posts with label science-series. Show all posts
Showing posts with label science-series. Show all posts
Thursday, April 19, 2007
Friday, February 16, 2007
Cars too want to loose Weight!
Keywords: Traction, Emission, Balance, Weight Transfer
How does your car handle on slippery Ice? One of the factors is:[1] Front wheel drives handle better than rear wheel drives on slippery roads as the weight of the engine is on the drive wheels, which helps to improve traction. How does weight affect traction? Though we won't see the exact science of it, we can get a peek at the factors that play a role in the story behind traction.
Medium sized cars weigh approx 600 lbs less than 25 years ago. Mid-sized Cars now weigh around the ballpark of 3000 lb.
A debate carried in an article, carries the following points:
"vehicle quality is a better predictor of safety than weight" says an University of Michigan physicist Marc Ross. "It turns out that relatively inexpensive light cars do tend to be unsafe but more expensive light cars are much safer and are as safe as heavier cars and SUV (sports utility vehicle) models," said Ross. "Analyzing statistical things is sort of funny," Ross said. "If you choose categories so you get cheap cars in your category, then the death rates go up. What we found is that the price of a car is a much better predictor of risk in traffic accidents than the weight of the car."
"No matter what you do, you cannot repeal the laws of physics," spokesman for the Insurance Institute for Highway Safety, Russ Rader said. "A larger, heavier vehicle is always going to be safer than a smaller, lighter vehicle. If you're looking at small cars vs. larger cars, small cars have twice as many occupant deaths as large cars." He noted, however, SUVs have a higher rollover risk and are disproportionately involved in single vehicle crashes.
Reducing the weight also brings with it other benefits: “A 40% decrease in car weight can result in a tremendous reduction in CO2 emissions,” says Ignaas Verpoest of Belgium’s Katholieke Universiteit Leuven.
How does weight interplay with traction? While driving, if you change direction towards your left, you get pushed to the right. This push transfers the weight on the left tires to the right. Your car has 60% of its weight (1800 lb) on the front tires and 40% on the back (1200 lb). So the car is unevenly loaded. When you make such a turn this push has a chance of pushing you over. The best protection against this comes by adding weight to the car: the greater the weight, the harder it is to transfer any weight from one side to the other. Some recommendations that come your way suggest placing weight on the rear ends to balance weight between the tires. Using a front-drive car, so the higher load on the front tires can be effectively countered.
There are other suggestions: Increasing the distance between the right and left tires (a person is more stable standing in "at ease" posture than in the "attention" posture). Also, reducing the Center of Gravity of the vehicle, or the "center of balance" of the vehicle (a Sumo wrestler or a Football player buckles down before taking a hit from the opponent). For something to think about until the next blog:
***For slippery ice, there is a lot more at play, on how the steering is used along with brakes, keeping off the gas pedals, etc. But these are things you do independent of the design of the car.
related: European cars are doing much better than U.S cars in mileage standards.
How does your car handle on slippery Ice? One of the factors is:[1] Front wheel drives handle better than rear wheel drives on slippery roads as the weight of the engine is on the drive wheels, which helps to improve traction. How does weight affect traction? Though we won't see the exact science of it, we can get a peek at the factors that play a role in the story behind traction.
Medium sized cars weigh approx 600 lbs less than 25 years ago. Mid-sized Cars now weigh around the ballpark of 3000 lb.
A debate carried in an article, carries the following points:
"vehicle quality is a better predictor of safety than weight" says an University of Michigan physicist Marc Ross. "It turns out that relatively inexpensive light cars do tend to be unsafe but more expensive light cars are much safer and are as safe as heavier cars and SUV (sports utility vehicle) models," said Ross. "Analyzing statistical things is sort of funny," Ross said. "If you choose categories so you get cheap cars in your category, then the death rates go up. What we found is that the price of a car is a much better predictor of risk in traffic accidents than the weight of the car."
"No matter what you do, you cannot repeal the laws of physics," spokesman for the Insurance Institute for Highway Safety, Russ Rader said. "A larger, heavier vehicle is always going to be safer than a smaller, lighter vehicle. If you're looking at small cars vs. larger cars, small cars have twice as many occupant deaths as large cars." He noted, however, SUVs have a higher rollover risk and are disproportionately involved in single vehicle crashes.
Reducing the weight also brings with it other benefits: “A 40% decrease in car weight can result in a tremendous reduction in CO2 emissions,” says Ignaas Verpoest of Belgium’s Katholieke Universiteit Leuven.
How does weight interplay with traction? While driving, if you change direction towards your left, you get pushed to the right. This push transfers the weight on the left tires to the right. Your car has 60% of its weight (1800 lb) on the front tires and 40% on the back (1200 lb). So the car is unevenly loaded. When you make such a turn this push has a chance of pushing you over. The best protection against this comes by adding weight to the car: the greater the weight, the harder it is to transfer any weight from one side to the other. Some recommendations that come your way suggest placing weight on the rear ends to balance weight between the tires. Using a front-drive car, so the higher load on the front tires can be effectively countered.
There are other suggestions: Increasing the distance between the right and left tires (a person is more stable standing in "at ease" posture than in the "attention" posture). Also, reducing the Center of Gravity of the vehicle, or the "center of balance" of the vehicle (a Sumo wrestler or a Football player buckles down before taking a hit from the opponent). For something to think about until the next blog:
***For slippery ice, there is a lot more at play, on how the steering is used along with brakes, keeping off the gas pedals, etc. But these are things you do independent of the design of the car.
related: European cars are doing much better than U.S cars in mileage standards.
Wednesday, March 08, 2006
As light leads us from Earth to the Sun
We may know our Sun (Our sun?) as it goes to sleep,
The sun went to sleep,or even as it is waking up
in a brilliant night dress,
That seemed to shorten,
as its light was seen less.
Until it had rested,
its weary warm head,
Within the cool blankets,
of moon glow, its bed!
-Linda A. Copp
The sun in the morning.But how well do we know our Sun? (this is probably the same problem every parent has with their child.) But keeping our discussion to the Sun; It is so scorching hot that we can't go near it, or peek into it to see what it is made of. This is not quite the truth.
Like a protective mother she rises and brings warmth to everything she touches.
Artists try to harness her beauty, scientists study to find her secrets.
Every being feels more alive when she is there sad when she is shrouded by a cloud.
She leaves each day with a promise to return that is never broken.
--By Bruce Patterson
Even as people reveal their true feelings when they become angry, any physical element shows its nature when it is very hot. The physics of it is: a hot body "feels" hot to us, and this heat comes to us as heat waves.
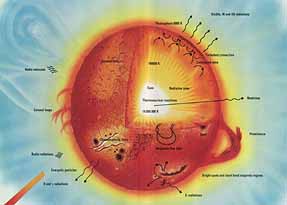
The light we see are waves of a particular length (or wavelength), and within them we see variations from blue (40 microns) to green to red (70 microns). One time when Newton passed white light through an optical prism, he found an image of a rainbow on the white screen nearby. This rainbow is the called "spectrum" of white light. You can also call it the signature of white light. Similarly when physical elements are heated at high temperatures, they begin to glow and emit waves, just like light, and that defines its own charateristic signature spectra. In the turn of the 19th century, enough progress had been made studying the signatures of Hydrogen and Helium at very high temperatures. (1000000 microns = 1 meter)
It was then possible to obtain signature of the heat waves coming from the sun and they began to show patterns that matched those of Hydrogen at high temperatures. This not only gave an idea of how hot the sun is (Its very hot!), but gave us an insight what is happening inside of it. The temperature on the sun is about 15 million degrees Celsius at its core.
At this temperature, as makers of the atom bomb also figured, many Hydrogen atoms can fuse into larger elements, thus producing a lot of energy. You see, the total mass of Hydrogen atoms is more than the mass of larger element produced. The remainder is released as energy in accordance with Einstein's relation that energy and mass are equivalent. We knew there was Hydrogen there and it was there at high temperatures. The patterns that matched were not exact, and what didn't match must have come from the heavier elements. You can also guess now that Sun has more hydrogen than of the heavier elements, as Hydrogen leaves heavier imprint on the signature of sun's heat waves than other elements.
So the theory was that Hydrogen fused to form Helium, and some Helium atoms further fused to form Carbon and so on. This theory is now backed by the signature patterns. We begin to appreciate how we have gone from visible light to make inference about the sun, which we can hardly look at. But, Sun is the Star of our solar system. Sun looks bright to us because it is so close to us. So the same story holds for all the stars that light up the night sky.
There are many unresolved questions. Will Hydrogen get exhausted? Remember some energy also comes from fusion of Helium atoms. How long will the sun last? How did it come to be in the first place. How old is it? There are many theories and each one was arrived at in its own peculiar way.
The sun went to sleep,
Within the cool blankets,
of moon glow, its bed!
Another day, Another time, Same blog.
Friday, February 17, 2006
Being on Cloud Nine
What would it be like if you were in the middle of a cloud? Can you Imagine millions of droplets of water floating around you? These droplets are very small, and they float around you without touching each other. This is how it would be to be among the clouds.[1] So is it all just water? Why don't they fall down? We can wonder what goes on up among those clouds, sitting here on the ground beneath our feet. (read my earlier blog to know about supercooling and crystallization).
The water droplets are so small that they can be kept afloat by small wafts of upward wind, more like a feather in the air that will float and float and float but will never fall to the ground. The droplets are kept from running into each other by the same air, which acts more like a foam cushion that keeps a high-jumper from bumping into the floor. You can imagine these droplets danced around by the wafts and yet not bumping into each other.
There is something more to clouds. Clouds are also very cold. So cold that it can be below -40 deg C (now you are having second thoughts about being in the cloud). You must have read my earlier blog and wonder: how can water remain unfrozen? The answer also lies in the same blog. For water to freeze, it needs points of crystallization. Up in the clouds these are provided by suspended particles (dirt) in the air. But as you can imagine, a cloud living so high is also far from the dust and dirt of the Earth![2] So it is very hard to find such dirt that can make crystallization to happen. So water droplets remain water droplets but at temperatures much below zero.
So all these droplets go as clouds from one place to another, to another. When do they ever come down as rain? Will they fall on me? The first question is easier than the second. So, we will answer the first.
As we understand it, supercool water (water below zero deg C) becomes unstable as the temperature decreases. Only the slightest disturbance is needed for it to freeze. So lower the temperature the less impurities are actually needed to cause the disturbance. After all, even in the clouds there are impurities, though not as many as on the surface of the Earth.
We know that warm currents and cold currents all have a thing going of their own, independent of the clouds. Where they come from, how they come, we don't care. But if enough cold winds come to make the supercool water droplet's temperature drop below -40 deg C, then crystallization can start. So droplets turn to ice and slowly the surface of ice (flake rather) becomes the source of further disturbance and the crystallization spreads through the cloud. There is an additional effect that happens here. There are wafts of wind blowing between the droplets. This air is rich in moisture (it contains water vapors) and on meeting ice, the moisture in the air, condenses and then freezes to form more ice.
We remember well that the droplets are floating away from each other. So, as the ice starts to form, the cloud does not become a block of ice but a cloud of snow flakes, all ready to fall. As they fall, they melt and drop as rain droplets, only much bigger ones than the ones in cloud. How so? The air added more ice to them from the moisture it was carrying, remember?
Most of the drops evaporate as the fall down, due to the resistance from the air, just the same resistance we feel when riding a bike on the road. The clouds contained in a cube of one kilometer for each side, weight about a few hundred tonnes, so heavier the clouds the more drops that actually fall down on it, heavier the rain.
So that is the story of rain. You may prefer to stand in the rain than to be among the clouds.
We can tweak science to create rain in places that don't traditionally receive rainfall. If you remember the relation between supercool water and disturbance needed for crystallization, we needed a lot of cold drafts because there was not much "dirt" up above. Now this is the reason why clouds pass by without raining. So what scientists propose is to introduce "dirt".
What I cannot explain well is that one kind of "dirt", when introduced, can actually trigger crystallization at a higher temperature than some other "dirt". Silver Iodide can do this at -20 deg C where as usual crystallization needs well below -40 deg C. The process of introducing Silver Iodide in clouds to create the rain formation artificially is called seeding the clouds. Silver Iodide needs to be sprinkled fairly well inside the cloud, so crystallization will be uniform. Or rather, the upward drafts must help mix silver iodide fairly well in the clouds. There are hundreds of factors that need to go right so rain can be formed from the clouds. Here is two important ones
1. If the cloud isn't precisely downwind from the target area, the rain will fall in the wrong place anyway.
2. If the seeding is done at the wrong time, or on the wrong cloud, it may cause "cratering," or large holes in the cloud that cause it to fall apart. So an errant effort can destroy the clouds that might otherwise produce rain.
[1] Its not all water. There is some ice too. But not so many that can induce crystallization. But mostly water.
[2] The reason why dirt and dust are less at higher levels is rather simple. Anything to rise to that level against gravity must have really small weight, or can be vaporised in gas form. Most dirt don't become gas, and as the threshold of weight becomes smaller, fewer kinds of dirt can qualify.
[3]Stratus, Cirrus, Cumulus are three common varieties of cloud.
I spare you from reading this rather longish article, by quoting some interesting excerpts from it:
Other seeding agents have been researched for the last 40 years, along with the importance of other conditions that help seeding to arrive at a better understanding of the process.
This resource provided the most material for the above write up. The following is another excellent very understandable discussion on everything there is to know about clouds. Recent News on Cloud seeding in Wyoming (Jan '06), and other ones in Australia, South Africa, Texas, and Israel.
Trivia: The Arctic ground squirrel hibernates at a body temperature below freezing--and yet doesn't freeze. The ground squirrel is able to do this because before hibernation it goes through a sort of internal purification that gets rid of any particle that might seed the freezing process. Liquids require such a particle (sometimes called a nucleus) in order to freeze. By purging itself the squirrel avoids becoming an ice cube, even if the temperature drops below freezing. (Link to Source Courtesy: Pradzie)
The water droplets are so small that they can be kept afloat by small wafts of upward wind, more like a feather in the air that will float and float and float but will never fall to the ground. The droplets are kept from running into each other by the same air, which acts more like a foam cushion that keeps a high-jumper from bumping into the floor. You can imagine these droplets danced around by the wafts and yet not bumping into each other.
There is something more to clouds. Clouds are also very cold. So cold that it can be below -40 deg C (now you are having second thoughts about being in the cloud). You must have read my earlier blog and wonder: how can water remain unfrozen? The answer also lies in the same blog. For water to freeze, it needs points of crystallization. Up in the clouds these are provided by suspended particles (dirt) in the air. But as you can imagine, a cloud living so high is also far from the dust and dirt of the Earth![2] So it is very hard to find such dirt that can make crystallization to happen. So water droplets remain water droplets but at temperatures much below zero.
So all these droplets go as clouds from one place to another, to another. When do they ever come down as rain? Will they fall on me? The first question is easier than the second. So, we will answer the first.
As we understand it, supercool water (water below zero deg C) becomes unstable as the temperature decreases. Only the slightest disturbance is needed for it to freeze. So lower the temperature the less impurities are actually needed to cause the disturbance. After all, even in the clouds there are impurities, though not as many as on the surface of the Earth.
We know that warm currents and cold currents all have a thing going of their own, independent of the clouds. Where they come from, how they come, we don't care. But if enough cold winds come to make the supercool water droplet's temperature drop below -40 deg C, then crystallization can start. So droplets turn to ice and slowly the surface of ice (flake rather) becomes the source of further disturbance and the crystallization spreads through the cloud. There is an additional effect that happens here. There are wafts of wind blowing between the droplets. This air is rich in moisture (it contains water vapors) and on meeting ice, the moisture in the air, condenses and then freezes to form more ice.
We remember well that the droplets are floating away from each other. So, as the ice starts to form, the cloud does not become a block of ice but a cloud of snow flakes, all ready to fall. As they fall, they melt and drop as rain droplets, only much bigger ones than the ones in cloud. How so? The air added more ice to them from the moisture it was carrying, remember?
Most of the drops evaporate as the fall down, due to the resistance from the air, just the same resistance we feel when riding a bike on the road. The clouds contained in a cube of one kilometer for each side, weight about a few hundred tonnes, so heavier the clouds the more drops that actually fall down on it, heavier the rain.
So that is the story of rain. You may prefer to stand in the rain than to be among the clouds.
We can tweak science to create rain in places that don't traditionally receive rainfall. If you remember the relation between supercool water and disturbance needed for crystallization, we needed a lot of cold drafts because there was not much "dirt" up above. Now this is the reason why clouds pass by without raining. So what scientists propose is to introduce "dirt".
What I cannot explain well is that one kind of "dirt", when introduced, can actually trigger crystallization at a higher temperature than some other "dirt". Silver Iodide can do this at -20 deg C where as usual crystallization needs well below -40 deg C. The process of introducing Silver Iodide in clouds to create the rain formation artificially is called seeding the clouds. Silver Iodide needs to be sprinkled fairly well inside the cloud, so crystallization will be uniform. Or rather, the upward drafts must help mix silver iodide fairly well in the clouds. There are hundreds of factors that need to go right so rain can be formed from the clouds. Here is two important ones
1. If the cloud isn't precisely downwind from the target area, the rain will fall in the wrong place anyway.
2. If the seeding is done at the wrong time, or on the wrong cloud, it may cause "cratering," or large holes in the cloud that cause it to fall apart. So an errant effort can destroy the clouds that might otherwise produce rain.
[1] Its not all water. There is some ice too. But not so many that can induce crystallization. But mostly water.
[2] The reason why dirt and dust are less at higher levels is rather simple. Anything to rise to that level against gravity must have really small weight, or can be vaporised in gas form. Most dirt don't become gas, and as the threshold of weight becomes smaller, fewer kinds of dirt can qualify.
[3]Stratus, Cirrus, Cumulus are three common varieties of cloud.
I spare you from reading this rather longish article, by quoting some interesting excerpts from it:
The researchers have found: Rainfall from seeded clouds lasted longer than rain from unseeded clouds, the rainfall covered a larger area, and total precipitation was higher, sometimes even doubled. And in many cases results began just 20 minutes after the seeding.
Mr. Bruinties is now in the United Arab Emirates conducting a three-month feasibility study to determine whether conditions there are right for a cloud seeding program. Remember, not all clouds can be seeded.
Only certain clouds, early in their formation, are useful, and timing is everything.
Other seeding agents have been researched for the last 40 years, along with the importance of other conditions that help seeding to arrive at a better understanding of the process.
This resource provided the most material for the above write up. The following is another excellent very understandable discussion on everything there is to know about clouds. Recent News on Cloud seeding in Wyoming (Jan '06), and other ones in Australia, South Africa, Texas, and Israel.
Trivia: The Arctic ground squirrel hibernates at a body temperature below freezing--and yet doesn't freeze. The ground squirrel is able to do this because before hibernation it goes through a sort of internal purification that gets rid of any particle that might seed the freezing process. Liquids require such a particle (sometimes called a nucleus) in order to freeze. By purging itself the squirrel avoids becoming an ice cube, even if the temperature drops below freezing. (Link to Source Courtesy: Pradzie)
Saturday, February 11, 2006
Warm Your Hands with Supercool Liquid
Keywords: Supercooling, Hand warmers, Crystallization.
If you hold on to the thread till the end, you might actually learn something. As a teaser: The title is sorta accurate. Read on to find out.
My friend and I went that evening to the sports store to do some ski gear shopping. As we left the store for another to do some price comparison, we entered into a chat with the guy at the counter. He said he was going to buy ski mittens instead of ski gloves. Minnesota cold meant mittens, which offer additional warmth for fingers (as they are next to each other). Mittens are also convenient for using hand warmers in them (these are heating pads inserted in the back of the gloves for additional warmth).
Hand Warmers: So our discussion turned to reusable hand warmers (see picture. the metal is at the bottom of the pouch). He showed us a small plastic pouch of liquid with a penny sized metal plate inside it. When the metal is bent, the liquid slowly starts to turn solid and white, and releases a lot of heat while solidifying.

The liquid, it turns out, is sodium acetate, which is a very specially suited liquid for use in hand warmers. The process of releasing energy it turns out is due to two other very special processes called supercooling and crystallization. These can be observed by performing experiments which you can do yourself either by buying an handwarmer pouch or even with water (you have to work extra hard for this).
Cooling and Crystallization: Supercooling is the principle that lies at the bottom of this heat release. Cooling, as we know it, is a process by which temperature of a liquid (think water) is reduced. As the temperature goes down slowly the liquid starts to turn into solid. What is really happening here is that liquid starts to go through a change in phase (from liquid to solid) as the temperature goes down.
In fact you can see that until the temperature reaches zero, the water remains water. No ice. When temperature reaches zero, the process called crystallization starts (nobody really understands how this works). Around an impurity or a surface with irregularities (even smooth surfaces have a small amount of irregularities), the water molecules find the source for some kind of a disturbance to their liquid state. At these crystallization points, water begins to crystallize (that is, becomes ice), right when temperature hits zero. Slowly as crystallization spreads, the surface of the ice thus formed becomes a natural site for further crystallization of the uncrystallized water that remains. Water placed in an ice cube box becomes ice much faster than if kept in a vessel: because the amount of surface irregularities for potential crystallization is larger in the ice box. The temperature at which the liquid crystallizes to solid is called the freezing point temperature. So that is the short primer on cooling and formation of ice for you.
Supercooling: If you cool a liquid very quickly and much below the freezing point temperature, the liquid can avoid crystallization and remain liquid. But, now it remains liquid at a temperature lower than the freezing point temperature. For this process, it is important that as the liquid is cooled it remains still and undisturbed. Even slight disturbances can provide a site for crystallization to start.
Crystallization and Heat: So when this supercooled liquid is disturbed, it immediately begins to crystallize. But then, when it crystallizes its temperature is restored to freezing point temperature. So all this energy that comes from crystallization (called latent heat) is released outside. If you must know, this kind of reaction where energy is released outside is called exothermic (exo=out, therma=heat).
Back to Hand Warmers: So this is what is happening with sodium acetate. It is naturally in the liquid form above 54 degC. On cooling, it will freeze at 54 degC. When I found this liquid pouch of sodium acetate at room temperature (25-30 degC), it is liquid at a temperature clearly below its freezing point temperature. But this pack is shaken, transported in trucks and sold in shops. How is it possible? Well, it turns out sodium acetate is special. It is very stable as a supercooled liquid. It needs heat to be supplied to disturb its condition and induce crystallization.
This is precisely the point of the metal piece inside. When the metal piece is bent, it produces a very small amount of heat; Heat that is good enough to make sodium acetate crystallize. Then sodium acetate freezes and restores itself to 54 degC. We find that it is heating up our hand because our outside body temperature is 25-30 degC.
So this is the cool story of how supercooling, crystallization, and sodium acetate come together to make our hands warmer.
Actually there is more unexpected stuff. You will have to wait until next week to find out, and this time you don't get to choose the liquid, but the metal.
Additional Notes:
[1] I never told you why the hand warmers were reusable. So what needs to be done with frozen crystallized sodium acetate is a careful process of boiling and cooling back to room temperature (remember that this is below the freezing point temperature of sodium acetate). So place this pouch in hot water and make sure every crystal is melted to liquid. Then let it cool without any disturbance. Since sodium acetate is quite stable it will supercool to room temperature nicely. Then you can reuse it by bending the metal plate again. You can do this experiment with water. However, though cooling water below zero degC (say to -2 or -3 degC) is possible, you have be sure that the container remians undisturbed throughout the experiment. And remember, water is not as stable in the supercooled state as sodium acetate.
Some >links<
How hand warmers work (scroll down), wiki on hand warmers and an experiment (.pdf file) with handwarmers. How supercooling might be useful in metal treatment and hydrogen fuel cells Understanding exothermic and endothermic reactions from pbs A demonstration of supercooling in water
Trivia:
[1] During the Korean war (circa 1924), Japanese soldiers mixed warming powder (a mixture of iron, water, cellulose, vermiculite, activated carbon and salt) with water to generate heat to help keep soldiers warm in the bitter cold of the wartime battlefield.
[2] Rusting of Iron produces heat, but the process is so slow that you don't notice it.
If you hold on to the thread till the end, you might actually learn something. As a teaser: The title is sorta accurate. Read on to find out.
My friend and I went that evening to the sports store to do some ski gear shopping. As we left the store for another to do some price comparison, we entered into a chat with the guy at the counter. He said he was going to buy ski mittens instead of ski gloves. Minnesota cold meant mittens, which offer additional warmth for fingers (as they are next to each other). Mittens are also convenient for using hand warmers in them (these are heating pads inserted in the back of the gloves for additional warmth).
Hand Warmers: So our discussion turned to reusable hand warmers (see picture. the metal is at the bottom of the pouch). He showed us a small plastic pouch of liquid with a penny sized metal plate inside it. When the metal is bent, the liquid slowly starts to turn solid and white, and releases a lot of heat while solidifying.

The liquid, it turns out, is sodium acetate, which is a very specially suited liquid for use in hand warmers. The process of releasing energy it turns out is due to two other very special processes called supercooling and crystallization. These can be observed by performing experiments which you can do yourself either by buying an handwarmer pouch or even with water (you have to work extra hard for this).
Cooling and Crystallization: Supercooling is the principle that lies at the bottom of this heat release. Cooling, as we know it, is a process by which temperature of a liquid (think water) is reduced. As the temperature goes down slowly the liquid starts to turn into solid. What is really happening here is that liquid starts to go through a change in phase (from liquid to solid) as the temperature goes down.
In fact you can see that until the temperature reaches zero, the water remains water. No ice. When temperature reaches zero, the process called crystallization starts (nobody really understands how this works). Around an impurity or a surface with irregularities (even smooth surfaces have a small amount of irregularities), the water molecules find the source for some kind of a disturbance to their liquid state. At these crystallization points, water begins to crystallize (that is, becomes ice), right when temperature hits zero. Slowly as crystallization spreads, the surface of the ice thus formed becomes a natural site for further crystallization of the uncrystallized water that remains. Water placed in an ice cube box becomes ice much faster than if kept in a vessel: because the amount of surface irregularities for potential crystallization is larger in the ice box. The temperature at which the liquid crystallizes to solid is called the freezing point temperature. So that is the short primer on cooling and formation of ice for you.
Supercooling: If you cool a liquid very quickly and much below the freezing point temperature, the liquid can avoid crystallization and remain liquid. But, now it remains liquid at a temperature lower than the freezing point temperature. For this process, it is important that as the liquid is cooled it remains still and undisturbed. Even slight disturbances can provide a site for crystallization to start.
Crystallization and Heat: So when this supercooled liquid is disturbed, it immediately begins to crystallize. But then, when it crystallizes its temperature is restored to freezing point temperature. So all this energy that comes from crystallization (called latent heat) is released outside. If you must know, this kind of reaction where energy is released outside is called exothermic (exo=out, therma=heat).
Back to Hand Warmers: So this is what is happening with sodium acetate. It is naturally in the liquid form above 54 degC. On cooling, it will freeze at 54 degC. When I found this liquid pouch of sodium acetate at room temperature (25-30 degC), it is liquid at a temperature clearly below its freezing point temperature. But this pack is shaken, transported in trucks and sold in shops. How is it possible? Well, it turns out sodium acetate is special. It is very stable as a supercooled liquid. It needs heat to be supplied to disturb its condition and induce crystallization.
This is precisely the point of the metal piece inside. When the metal piece is bent, it produces a very small amount of heat; Heat that is good enough to make sodium acetate crystallize. Then sodium acetate freezes and restores itself to 54 degC. We find that it is heating up our hand because our outside body temperature is 25-30 degC.
So this is the cool story of how supercooling, crystallization, and sodium acetate come together to make our hands warmer.
Actually there is more unexpected stuff. You will have to wait until next week to find out, and this time you don't get to choose the liquid, but the metal.
Additional Notes:
[1] I never told you why the hand warmers were reusable. So what needs to be done with frozen crystallized sodium acetate is a careful process of boiling and cooling back to room temperature (remember that this is below the freezing point temperature of sodium acetate). So place this pouch in hot water and make sure every crystal is melted to liquid. Then let it cool without any disturbance. Since sodium acetate is quite stable it will supercool to room temperature nicely. Then you can reuse it by bending the metal plate again. You can do this experiment with water. However, though cooling water below zero degC (say to -2 or -3 degC) is possible, you have be sure that the container remians undisturbed throughout the experiment. And remember, water is not as stable in the supercooled state as sodium acetate.
Some >links<
How hand warmers work (scroll down), wiki on hand warmers and an experiment (.pdf file) with handwarmers. How supercooling might be useful in metal treatment and hydrogen fuel cells Understanding exothermic and endothermic reactions from pbs A demonstration of supercooling in water
Trivia:
[1] During the Korean war (circa 1924), Japanese soldiers mixed warming powder (a mixture of iron, water, cellulose, vermiculite, activated carbon and salt) with water to generate heat to help keep soldiers warm in the bitter cold of the wartime battlefield.
[2] Rusting of Iron produces heat, but the process is so slow that you don't notice it.
Subscribe to:
Posts (Atom)